PD-1 inhibitors have emerged as an important class of immunotherapy drugs and several agents, such as nivolumab, are now FDA approved for the treatment of various malignancies, including classical Hodgkin lymphoma (cHL). By blocking the interaction of PD-1 with its ligands, PD-1 inhibitors unleash T-cells to attack neoplastic cells, but also interfere with immune self-recognition of healthy tissue. PD-1 inhibitors can thus lead to toxicity by immune attack of several organ systems.
Less frequently, an exaggerated immunological response known as cytokine release syndrome (CRS) can develop. Unhindered T-cell activation leads to activation of bystander immune (dendritic cells, macrophages) and non-immune cells (endothelial cells) and ultimately massive release of a range of cytokines . CRS can be life-threatening, leading to hemodynamic instability, liver dysfunction, DIC and multiorgan failure.
A limited number of CRS cases in patients treated with immune checkpoint inhibitors (ICIs) have been reported to date. Almost all cases have early-onset CRS with a median of 4 weeks from treatment initiation. Late-onset CRS has been documented in only one case of a patient with melanoma treated with nivolumab. No obvious risk factors for CRS have yet been identified in the context of ICIs, although high burden of disease has been linked to higher rates and severity with other immunotherapies, such as bispecific antibodies and CAR-T cells, which can explain the early-onset of the syndrome.
We now report another case of late-onset CRS with nivolumab, this time in a patient with cHL and HIV. CRS coincided with loss of HIV control due to a period of non-compliance with anti-retroviral therapy (ART), which likely contributed to the development of cytokine storm.
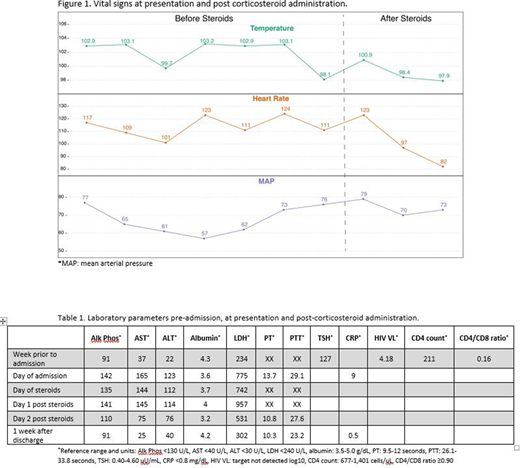
A 28-year-old female with HIV infection on ART and chemotherapy-refractory stage IV cHL on cycle 11 of nivolumab, with a favorable response, was admitted with worsening fatigue, subjective fevers, diarrhea and mild headaches. She was febrile, hypotensive and tachycardic (Figure 1). Physical exam was essentially normal. Laboratory work-up showed new transaminitis, elevated LDH and slightly prolonged PT; she was not neutropenic. She was hypothyroid due to non-compliance with levothyroxine for nivolumab-related hypothyroidism. Newly elevated HIV VL along with dropping CD4 count and CD4/CD8 ratio were noted (Table 1). She reported recent non-compliance with ART.
She continued to have unremitting high fevers with persistent hypotension after 24 hours of IV hydration, anti-pyretics and broad-spectrum antibiotics. CRS was suspected. Adrenal insufficiency and hypophysitis were ruled out. CRP was elevated to 9mg/dl. She was started on methylprednisolone 1 mg/kg/day.
She clinically improved within 5 hours of first steroid dose (Figure 1) and was discharged 2 days later on a 2-week-long steroid taper. Re-challenge with nivolumab approximately 1 month later, with undetectable HIV viral load, led to no recurrent events. She remains on treatment and in complete metabolic remission after 2.5 years of therapy.
HIV infection leads to disturbed T-cell homeostasis, generating a systemic inflammatory environment and shifted profiles of inflammatory markers. Untreated infection leads to massive depletion of both HIV-infected and uninfected bystander CD4 cells, inverted CD4/CD8 ratio, impaired CD8 T cell function with increased expression of immune checkpoint proteins (such as PD-1) and elevated plasma levels of cytokines including INF-g, TNF and IL-6. It is possible that loss of HIV control in our case, promoted an evolving state of immune dysregulation with low CD4/CD8 ratio, increased PD-1 expression and elevated cytokines, which under the pressure of PD-1 inhibition led to an uncontrolled immune stimulation, culminating in a cytokine storm.
Initial ICI cancer trials have largely excluded HIV patients, but their safety is increasingly recognized in this population. Our case highlights the nuances around safety and efficacy of immunotherapy in HIV. Nivolumab was highly efficacious and well-tolerated while ART maintained viral suppression. CRS coincided with loss of virologic control and did not recur upon re-challenge, once HIV infection was controlled. We conclude that continuous viral suppression may be key for the safe implementation of ICIs, and likely other forms of immunotherapy, in patients with cancer and HIV.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.